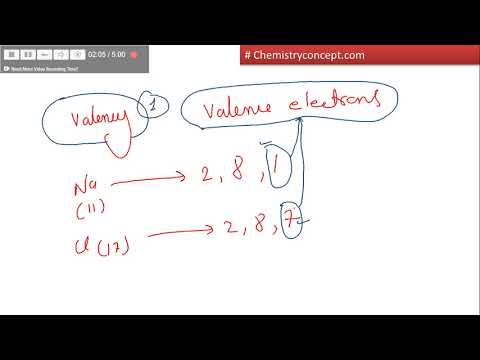
Valence electrons are the electrons present within the outermost shell of an atom. That is, we can finally say that there are electrons equal to the atomic number within the iron atom. From the periodic table, we see that the atomic variety of iron is 26.
For impartial atoms, the variety of valence electrons is equal to the atom’s primary group number. Formula to calculate valence electrons for impartial atoms. This also means that when looking at a bunch number, exclude the transition metals. They are located within the block in the midst of the periodic desk.
Antraniks Good Core Program
The electrons revolving in the outer most orbit of a atom has highest power degree. Valence electrons are loosely hooked up to the nucleus. Therefore, less amount of power is required to pull an electron from the outer most orbits.
- There are many alternative ways to search out out the valency of an atom which reflects the power of an atom to bond with other atoms.
- According to the Bohr-Bury system, an atom’s outermost shell can maintain as a lot as 8 electrons.
- However, these valence electrons don’t contribute to the electron protect.
- From the electron configuration of oxygen atoms, we see that the second orbit of oxygen is the last shell and there are a complete of six electrons in the last orbit.
- Valence electrons attract one another, they also either need to switch between molecules or share between molecules.
They will be capable of reply questions such as ” the variety of these electrons in cl- ion are? ”, or calculate the number of iodine outer-shell electrons of fluorine outer-shell electrons by referring to the periodic desk. Know what are ionic bonds and covalent bonds to grasp the role of valence electrons in bond formation. Use the rows of the table as orbital shell shortcuts. You can use this as a shortcut to discover out how many valence electrons an element has — just begin from the left facet of its interval when counting electrons. Since it’s the sixth element from the left in the fourth interval , we know that the outer fourth shell has six electrons, and, thus, that Selenium has six valence electrons.
That is, the iron atom has a complete of twenty-six electrons. While the number of valence electrons will increase by one over time, the variety of shells remains constant. The number of shells round an element’s nucleus is indicated by the interval number .
Now we are going to learn how to determine the valence electron of iron. To find valence electrons utilizing a period table, first see if your atom is a transitional metal, that are the elements in the middle rectangle of the table. If the atom is outdoors this block, find its group quantity along the boron valency top of the table. The ones digit in the group number is the variety of valence electrons. To remedy without a periodic table, discover the electron configuration of the component and depend the electrons into 1 group of two, after which into shells of eight. The number within the last group is the quantity of valence electrons.
Why Are Valence Electrons Important?
Both valency and valence electrons are applied for any chemical factor. This is a color-coded table made up of many different squares that lists all the chemical parts identified to humankind. You can often find these inside the cover of chemistry textbooks. There is also an excellent interactive table out there online right here. The periodic desk is divided into four blocks by electron configuration.
Thereafter the variety of electrons within the outermost shell gives the total number of valence electrons in that element. The most reactive metallic parts, such as sodium and potassium, are found in group 1. As a outcome, group 1 components have single valence shell electrons that can readily be misplaced to supply a optimistic ion. As a end result, it solely has one electron to lose, making it easier to connect and extra reactive. Because the metals in group 2 have two valence electrons of their valence shell, they have to lose two valence electrons to produce a constructive steel ion.
It only seems when there are double or triple bonds asymmetrically positioned. We can even find the valency of silicon with the assistance of the periodic desk. As silicon belongs to group 14 together with carbon , germanium , tin , lead , and flerovium . You must know how to discover the variety of valence electrons in a molecule to find a way to sucessfully draw Lewis Structures. Using the idea of proton-electron sights college students will see trends of atomic radius across rows of the periodic table. Do not want to achieve or lose electrons to realize an octet.
Browse other questions tagged electrons physical-chemistry molecules or ask your personal query. Valence electrons relate to the place of components inside the groups and durations of the periodic desk, and also their place within blocks. The placement of elements and electrons can differ and in reality bonds are a blend of all these totally different attainable structures. If you only have single bonds, you can’t present resonance although.
How Many Valence Electrons Does Galliumga Have?
This article discusses intimately the means to easily decide the variety of valence electrons in an element. Hopefully, after reading this article you will know the small print about this subject. Because they are in the highest power stage, they’re typically essentially the most concerned in chemical reactions since they’re the easiest to transfer. The outer-shell electrons are transferred between the atoms to deliver stability to the atom.